ESR1: Modulation of antioxidant status in dysfunctional dopaminergic neurons
Nicoló Bonora (Universidad de Salamanca)
Supervisor: Juan Pedro Bolaños
Project summary
The vast majority of previous studies on ROS-mediated cell signalling have used a strategy based on increasing ROS, either by exogenous or endogenously supplied ROS; we believe that this approach might mask the beneficial effects of mROS, and therefore we plan the alternative approach to down-modulate basal mROS in specific brain cells using a transgenic mice line expressing mitochondrial-tagged form of catalase (mCAT). With the expected results, we aim to conciliate the current controversy on the pathological or physiological functions of mROS, and to elucidate the molecular targets responsible for the metabolic adaptations of neurons to stress, aiding the rational design of new therapeutic strategies against neurodegeneration.
The specific aims of the project are:
- to investigate whether endogenous mROS constitutively up-regulate the Nrf2 transcription factor and his target genes astrocytes
- to assess whether energy antioxidant status and survival of neurons depends on mROS-induced gene expression in astrocytes
- to identify new compounds that, by activating Nrf2 in astrocytes, promote neuronal survival
Aim 1: using mice primary astrocytes expressing mCAT ubiquitously, we down-modulate endogenous mROS. First of all, we will verify that the mCAT decrease the mROS, using fluorimetric techniques. Then the stabilization of Nrf2 will be studied, as their respective target genes (mRNAs) and protein abundances. Their functional effects will be evaluated by determining the activity of antioxidant enzymes and signs of stress (apoptosis and mitochondrial bioenergetics)
Aim 2: mCAT will be expressed in astrocytes, both in primary culture and in vivo, and its effects on neurons will be evaluated in astrocytic-neuronal co-cultures and in vivo. For the in vitro approach we will co-culturing astrocytes expressing mCAT ubiquitously with neurons wild type; for the in vivo approach, a Cre-recombinase mCATfloxed mouse will be generated using the Rosa26 strategy and crossed with the mice expressing Cre governed by the astrocytic-specific GFAP (Glial-Fibrillary Acidic Protein) promoter (GFAP-mCAT). We will analyse both in vitro and in vivo the oxidative status, signs of stress (apoptosis, mitochondrial bioenergetics) in neurons to assess if the down modulation of mROS in astrocytes have some detrimental consequences on neural cells.
Aim 3: in collaboration with Biomar Microbial Technologies (Léon) we will screen new compounds, from a pre-selected set, that can specifically activate Nrf2 and their target genes in astrocytes. The possible neuroprotection of these compounds will be evaluated in astrocytic-neuronal co-culture. Currently, we are characterizing the astrocytes expressing mCAT ubiquitously; in particular we verified that the model works and the mROS decrease. As a consequence we saw a decreased stabilization of Nrf2 and we are now evaluating the transcription of its respective target genes and protein abundances. In parallel, we are crossing and breeding the mice line GFAP-mCAT to start as soon as possible with the in vivo experiments.
Results [top]
Aim I: One of the stated goals of the TINTIN project is the generation of a knock-in mouse model harbouring a mitochondrial form of the
catalytic subunit of the enzyme catalysing the first rate-limiting step of glutathione biosynthesis (Glutamate-Cysteine ligase, catalytic
subunit, mitochondrial-tagged; mGCL) using the Rosa26 strategy by flanking a STOP signal by Cre recombinase LoxP sites, in order to try
to specifically down-modulate mROS in brain cells. In fact, expression of mGCL should decrease mROS in the cells harbouring this protein.
However, after submitting the final Annex I, our laboratory found that, in order to efficiently detoxify mROS, mGCL-produced γ-glutamil-
cysteine (i..e, the efficient mROS-detoxifying cofactor) is oxidized and re-reduced thereafter at the expense of NADPH, a cofactor mainly
produced by glucose metabolism through the pentose-phosphate pathway. Therefore, mROS detoxification using the mGCL model may alter
glucose metabolism, which is one of the phenotypes that we would like to investigate.
Accordingly, we decided to improve the strategy
by designing a mitochondrial-tagged form of catalase (mCAT), an enzyme that detoxifies hydrogen peroxide in a clean and direct way that
does not need to consume glucose or alter any other known metabolic pathway, like mGCL does. Our mCAT construction contains a mitochondrial
tag (C8), the Catalase sequence and an HA tag, preceded by a strong CAG promoter and a STOP signal flanked by two LoxP sites and (Figure 1).
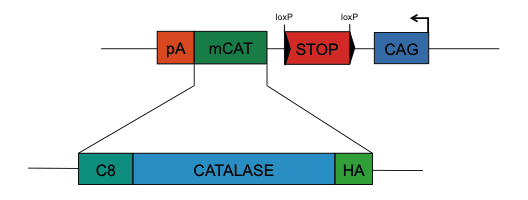
Figure 1: mCAT construction
Outputs [top]
In progress.
Training [top]
(i) Local level
I. Course for operating with living animals, category B (Salamanca, February 2015)
II. Course "Oral presentation and communication: strategies and new technologies"
(Universidad de Salamanca, available on September)
(ii) Network level
I. NMR Mini Boot Camp of BioBank Analyses and Metabolomic Transformation (Dublin, November 2014):
II. Fluorescence and electron microscopy imaging of cells (Dublin, May 2015)
III. Training in mitochondrial and cellular respiratory physiology (Dublin, May 2015)
IV. Neuronal Cell Metabolism (Dublin, May 2015)
Outreach [top]
Participated in the Open Day in the Institute of Functional Biology and Genomics (IBFG) which it took place on
May 7th, 2014